Российские исследователи провели анализ разнообразия белковых роторов.
The article has been published in the journal PROTEINS: Structure, Function, and Bioinformatics. ATP synthase utilizes membrane potential to synthesize adenosine triphosphate (ATP) molecules, the universal "fuel" of living systems. This enzyme can be likened to an electric motor: it has a stationary part — the stator, and a rotating part — the rotor, also known as the c-ring. Ions transported by ATP synthase across the membrane facilitate the rotation of the rotor and the synthesis of ATP by the stator. The rotor itself is composed of repeating units (c-subunits), the number of which determines how many ions will pass through the membrane in one rotation and what potential is necessary for the protein to function.
There are known ATP synthases that contain between eight and 17 c-subunits. X-ray crystallography or cryo-electron microscopy has been employed to study them. However, advancements in machine learning techniques have led to the development of several groundbreaking approaches, including those for predicting protein structures. The developers of the most renowned of these — AlphaFold — were awarded the Nobel Prize in Chemistry in 2024. Nonetheless, standard AlphaFold does not perform well with large protein complexes, including ATP synthases.
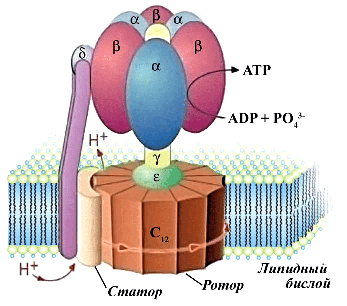
In their study, researchers from MIPT proposed a new method based on AlphaFold that confidently predicts the number of subunits in protein complexes with rotational symmetry. For known data, the correlation between predictions and experimental values exceeded 90 percent. Furthermore, the approach proved to be very rapid, enabling the estimation of subunit counts in ATP synthases across a wide variety of living organisms.
“The most unexpected aspect of our findings is the prediction of the existence of microorganisms in nature with very large c-rings in ATP synthases, which may have up to 27 repeating subunits! Previously, experiments had shown a maximum of 17 c-subunits. Importantly, molecular dynamics simulations confirm the results of these predictions,” says Stepan Osipov, the first author of the published work and a graduate student at the Landau Institute for Theoretical Physics at MIPT.
Why does nature require such unusually large c-rings when more "standard" rotors also exhibit high efficiency? The answer to this question is closely related to the environments in which these organisms thrive. They may exist at high temperatures or in harsh conditions where they constantly lose ions through their membranes. Alternatively, it could be that the membrane potential is low — in which case, a high number of repetitions in the c-ring is intended to better "capture" rare protons or sodium ions. Such large rotors might "consume" more protons per rotation but allow for ATP synthesis at significantly lower transmembrane potentials. To verify these hypotheses, experimental studies of actual microorganisms, which are predicted to have such unusual c-rings in ATP synthases, will be necessary.
“This also means that new opportunities arise in many areas of biotechnology. For instance, if we learn to engineer molecular 'motors' with specific parameters, we can deliberately adjust the efficiency of energy exchange in cells and create microorganisms for the production of various compounds in environments where traditional strains cannot survive,” comments Alexey Vlasov, senior researcher and acting head of the laboratory of molecular cell biology and optogenetics at MIPT.
“Our work highlights the importance of computational algorithms based on machine learning and artificial intelligence in modern structural biology, as well as opens new avenues for discovering interesting proteins in genomic data and engineering new proteins with desired properties,” concludes Ivan Gushchin, executive director of the Center for Research on Molecular Mechanisms of Aging and Age-Related Diseases at MIPT.
The work was supported by the Russian Science Foundation and the Ministry of Science and Higher Education of Russia.