The process of melting high-entropy carbonitrides has been studied using neural networks.
The results of the study published in the journal Scientific Reports indicate that high-entropy carbonitrides can be utilized as promising materials for protective coatings on equipment operating under extreme conditions—such as high temperature, thermal shock, and chemical corrosion.
“In this new research, we employed interatomic interaction potentials based on deep neural networks to model the structure of high-entropy carbonitride y (TiZrTaHfNb)CxN1−x in both solid and liquid states. This enabled us to predict heating and cooling temperatures depending on nitrogen content, determine the melting temperature, and analyze the structure-property relationship in terms of interatomic interactions. An increase in nitrogen content leads to a rise in melting temperature, which is associated with changes in the relative stability of the liquid phase compared to the solid phase upon nitrogen addition,” stated the study leader Alexander Kvashnin, a professor at the Skoltech Energy Transition Project Center.
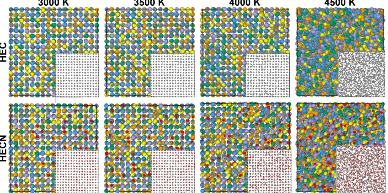
A group of scientists developed a new training procedure for the DeepMD potential to model the melting and crystallization processes of the material TiZrTaHfNbCxN1-x, which subsequently allowed them to calculate its melting temperature. The potential was trained on trajectory data obtained from molecular dynamics simulations using density functional theory, achieving high predictive accuracy.
This approach aims to enhance the capabilities of classical molecular dynamics modeling, enabling precise simulation and analysis of the melting process while predicting the melting temperature not only for high-entropy carbonitrides but also for other complex multi-component materials.
The authors identified the maximum melting temperature for the composition (TiZrTaHfNb)C0.75N0.25 as 3580±30 K. By adding nitrogen, the melting characteristics of high-entropy compounds can be improved—this will allow for modifications in the thermophysical properties of functional and structural materials.