A new Chinese flu medication surprisingly showed significant benefits for men, but not for women.
Authors of recent medical studies have discovered numerous new methods to combat well-known diseases. For instance, leprosy was successfully treated using an antibiotic typically employed for tuberculosis. Additionally, cancer was addressed through the camouflage of tumor cells as pig organ cells.
Experts from several universities and hospitals in China developed and tested a flu medication (which targets four viruses: Alphainfluenzavirus, Betainfluenzavirus, Gammainfluenzavirus, Deltainfluenzavirus) that demonstrated high effectiveness. A scientific article about it was published in the journal Nature Medicine.
Suraxavir marboxil is an antiviral drug that inhibits the polymerase acid protein RNA polymerase, which is the enzyme responsible for synthesizing nucleic acid polymers. It was administered to outpatient flu patients without severe complications for two days after symptom onset, while the control group received a placebo. The study included 591 individuals aged five to 65, with two-thirds receiving the experimental medication and one-third taking the "dummy" treatment.
The primary measure of the drug's effectiveness considered by the researchers was the time required for alleviating flu symptoms. Scientists monitored how quickly the participants' body temperature returned to no higher than 37.2°C, as well as tracking other symptoms: cough, sore throat, headache, nasal congestion, fever or chills, muscle or joint pain, and fatigue.
The median waiting time, which represents the value that divides an ordered data set into two equal parts, was significantly shorter in the group receiving suraxavir marboxil compared to the control group. In the former, the time was 42 hours, while in the latter, it was 63 hours.
The viral load— the amount of virus in the patient's material— changed more rapidly for those receiving the new medication than for those taking the placebo. However, suraxavir marboxil was associated with more adverse effects: mild or moderate side effects were experienced by 28.4% of those taking the drug, compared to 23.3% of those treated with the placebo.
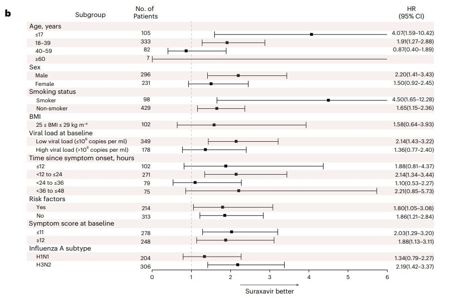
Moreover, the effect of the new medication was more pronounced in men than in women. Although in some aspects suraxavir marboxil affected patients of different genders similarly (for example, the viral load on the second day of treatment decreased in men just as it did in women), some metrics (average waiting time until symptom relief) were less significant for female participants.