Scientists have discovered the chemical reactions that played a crucial role in the emergence of life on Earth.
When life on Earth was just beginning to emerge, hot springs and minerals containing iron sulfide (FeS) could have played a crucial role in its origin. This inorganic compound is known for its ability to accelerate chemical reactions and resembles coenzymes that participate in modern biochemical processes.
That is why in 1988, German lawyer and chemist Günther Wächtershäuser proposed the iron sulfide world hypothesis, suggesting that the very first organism on Earth originated in a volcanic hydrothermal flow (at high temperatures (100 °C) and high pressures) on the surface of iron sulfide crystals. This form of life with a complex chemical structure utilized catalytic centers based on transition metals such as iron and nickel (and possibly zinc, cobalt, manganese, and tungsten).
However, the role of iron sulfides under the conditions present in ancient hot springs has remained largely unexplored. Now, the authors of a new study, published in the journal Nature Communications, have proposed that iron sulfides facilitated the conversion of gaseous carbon dioxide into prebiotic organic molecules.
A team of scientists led by Jingbo Nan from the Nanjing Institute of Geology and Palaeontology (China) and Martin Van Kranendonk from the University of New South Wales (Australia) modeled the environment of ancient hot springs under laboratory conditions. This allowed them to closely examine the role of iron sulfides in carbon fixation during the initial stages of life's evolution.
Using an anaerobic chamber and employing gas chromatography (a physicochemical method for separating substances), the researchers synthesized a series of nanosized iron sulfides from macinavite, including pure iron sulfide and iron sulfides enriched with common elements from hot springs (manganese, nickel, titanium, and cobalt).
The scientists found that at a temperature of 120 °C, manganese-enriched iron sulfide increased methanol (CH₃OH) production fivefold compared to pure iron sulfide. It is worth noting that methanol is a simple organic molecule that could serve as a building block for more complex compounds.
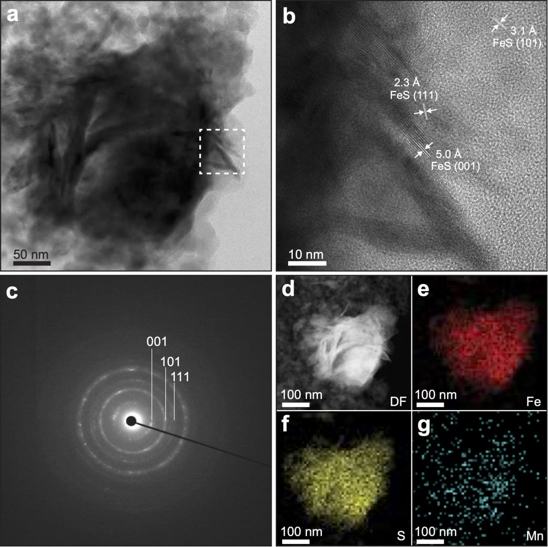
The catalytic activity of iron sulfide was also enhanced by exposure to ultraviolet and visible light. This is significant because, at the dawn of life, solar radiation was likely more intense and played a crucial role in chemical processes on the planet's surface.
Thus, experiments conducted under laboratory conditions and theoretical calculations showed that the intermediate product arising from the reverse reaction of the water-gas shift was carbon monoxide (CO), which subsequently converted into methanol.
This discovery will help to understand how simple inorganic compounds could be transformed into organic molecules without the involvement of living organisms, and explain the emergence of the first molecules—precursors of life on Earth, as well as identify the conditions that facilitated this process. The results may be useful for astrobiologists in the search for life on other planets with similar conditions.