Skoltech explained the significance of a key ingredient in the electrolytes of lithium-ion batteries.
The study was published in the Journal of Materials Chemistry A. During the early stages of lithium-ion battery commercialization, researchers encountered the issue of corrosion of the graphite anode: propylene carbonate (PC)-based electrolytes interacted well with metallic lithium but proved to be extremely aggressive towards graphite.
This hindered the use of graphite electrodes until ethylene carbonate (EC) was proposed as an alternative solvent in the electrolyte formulation. Despite the similarities between the molecules of EC and PC from an electrochemical perspective, they behave differently concerning graphite anodes. The nature of this difference and the behavior of the "magic solvent" EC have been the subject of numerous studies and discussions over decades, yet scientists still do not have a consensus.
Moreover, this question is not purely theoretical, and the answer will be significant in battery design, particularly regarding solvent selection in the electrolyte.
In their article, senior researcher Sergey Luchkin and leading manufacturing engineer Yegor Pazhetov from the Skoltech Energy Technologies Center hypothesized that the presence of EC in the electrolyte results in the formation of a thin layer of very viscous liquid on the surface of graphite. This layer protects graphite from corrosive delamination. Subsequent experiments confirmed that such a layer indeed forms in EC-based electrolytes and is absent when using PC.
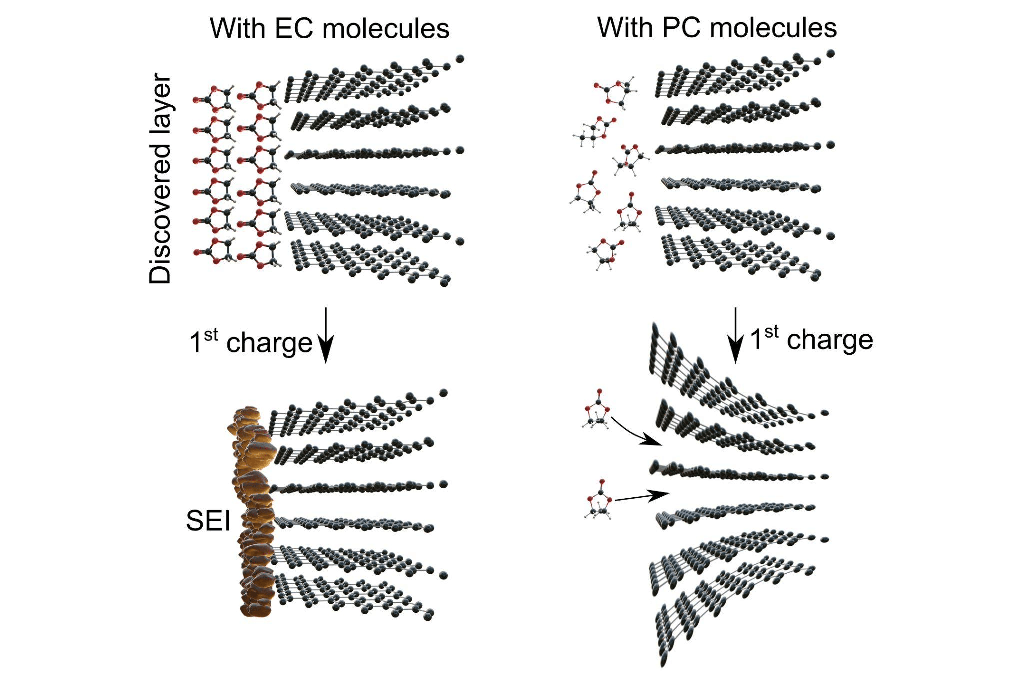
Interestingly, this viscous liquid layer appears before the formation of a crucial component of the lithium-ion battery—the so-called solid electrolyte interphase (SEI)—and therefore should influence its formation process. The solid electrolyte interphase is a thin film of solid electrolyte that forms on the anode surface during the initial charge and discharge of the battery at the factory. This film prevents both the degradation of the graphite anode and the electrolyte's decomposition—a process that deteriorates the device's performance.
This new perspective on interfacial processes in lithium-ion batteries opens up new avenues for understanding the relationship between electrolyte composition and interfacial dynamics between the electrolyte and anode, which is critical for developing more stable and efficient batteries.
The approach proposed in the study is applicable not only to widely used lithium-ion batteries but also to the emerging sodium- and potassium-ion batteries. The issue of solid electrolyte interphase formation is also relevant for these energy storage technologies. The work of the Skoltech researchers provides deeper insights into how the physical properties of electrolyte components affect interfacial processes, potentially accelerating innovations in energy storage.
The research highlighted in the press release is supported by a grant from the Russian Science Foundation.